Walking in Mitsunobu's Shoes - Part 1: Meet your first 'Designed' reaction
Those of you love to do some organic conversions now and then (Like 'Conversions and espresso' sounds like 'Netflix and pizza'), must have felt the frustration when you had to face the challenge of removing an OH leaving group. 'Cause that group is stubborn as hell. You can almost never remove an OH group by a direct nucleophilic substitution at the carbon center.
So, what do you do? There must be ways to avoid this, and there are, which you must be knowing if you have faced this problem several times. There are, roughly speaking, 3 possible strategies … or Tricks to remove an OH.
If SN2 doesn’t work, well, try something else:
Most nucleophiles have, to some extent, a basic nature. So, the conditions in which SN2 reactions commonly works are basic, neutral at most. But if that’s not going to work then we can change the conditions from basic to acidic. In an acidic medium, the OH group will take up a proton, becoming OHH…
Well, H2O; which is a fantastic leaving group (stable as hell, as you can see around). So an acid catalyzed reaction followed by some other nucleophile can make OH go away.
Problem: an Acidic condition often means aqueous conditions and therefore there is a competition between the nucleophile of your interest present in the solution and water itself (i.e., reversibility of the process).
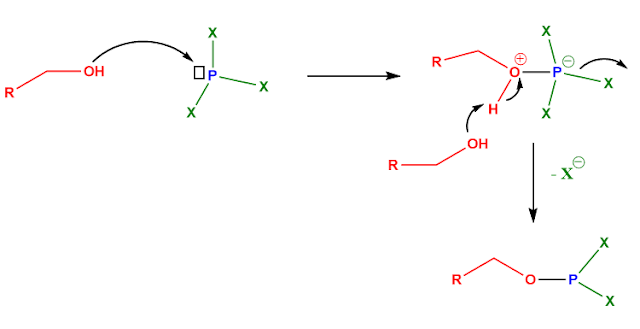
2 Using trihalophosphines:
The idea is to attach something to the OH group that makes it a leaving group.
Trihalophosphines (PX3) is one such group. The phosphorus has vacant 3d orbital and also is good electrophilic site due to the electronic pull of the three halo groups. So, the OH attacks the P center (donates to its LUMO) and phosphorus bears a negative charge on it, immediately one halide ion leaves, making phosphorus neutral again.
The next thing is a nucleophilic attack by the ‘exterminated’ halide ion on the carbon attached to the oxygen, which now is a part of a good leaving group.
Problem: well, keeping aside the lab-problems, theoretically the leaving group is not so good here. Had there been a + charge on the phosphorus it would have been a much better leaving group. We will come to this issue while designing our strategy.
3 Use Tosylate, Mesylate:
Well, this method is pretty much well established and I guess most of you would follow this one only. There is nothing wrong with it especially if all steric factor is taken care of. Though, Ts and Ms are quite good leaving group due to resonance stabilization it also makes them vulnerable to all nucleophiles present in the solution.
Comment: Note that, we have discussed mainly SN2 type of removal of the OH group. So, it goes without saying that it is not going to work for 3o alcohols. For them, we have to follow something along SN1 pathway.
Design Your Reaction:
Now, that we have an overview of the processes (I know there are many more processes that removes OH, but for simplicity and to elucidate the significance of Mitsunobu reaction these will do), let’s move to a deductive approach to solve the remove-that-OH issue.
You are coming up with your own plan, so what would you first see from the above 3 methods. Well, the first method is entirely changing the reaction condition (from basic to acidic), so that’s not very challenging; next, the Ts and Ms groups are pretty much already existing trump cards, rather the second method has a lot more chance of modification.
It is time to generalize the idea of the 2nd method:
Strategy:
· Attach OH group to some other species/groups
· Such that the whole thing, now including the oxygen of the alcohol, becomes an excellent leaving group.
Let’s call this the “Anchoring Strategy”
Now, to attach OH to some group, that group must be a good electrophile (like PX3 there). But, as we already mentioned in the problem of the 2nd method above, we need a positive charge on the phosphorus to make it a much better-leaving group. You see, that is very different from what happened there, P gained a negative charge due to being attacked by alcohol, and whenever any PR3 group is present in the neutral form, upon attack by OH, it’s going to have a negative charge on top.
So, we have two things to take care of in hand:
- Attach OH to a good electrophile, some phosphine species
- After attaching there should be a +ve charge on P.
Fortunately, sometimes two problems can ‘cancel’ each other out. The two above criteria can simultaneously be fulfilled if we extend this method of “anchoring strategy” one step further. The phosphine species is going to ‘anchor’ to some other group first, making it a nucleophile (Here comes the beauty behind using phosphine species, phosphorous has both a lone pair and vacant 3d orbital, making it an amphoteric species in context). So, the ‘neutral’ PR3 species is going to attack another group, let’s call it ‘the blob’ ( a fancy name for a chemical reagent/species), and upon attacking it, phosphorus is going to have a positive charge on top (note that, no R group on P should leave or add, which will change the charge on P, to make sure that no R group leaves, make R an alkyl group, like phenyl). Then, the alcohol will attack the positively charged phosphorus center, making the blob a leaving group.
Now, grasp the “2nd level” anchoring here. In the 1st level i.e., strategy, we were concerned with an anchoring group to which OH will attack and then the whole thing will become a leaving group. To make that a good leaving group, we need a +ve charge on P, to do that, we make it attack the blob, some electrophilic group which also should be a good leaving group and ready to be removed upon the attack of OH on positively charged P. Then, mission accomplished, we have the oxygen attached to a positively charged phosphine group.
But, there are other issues as well, its good not to hide the problems but rather show the complete hand on the table. You must have seen that the alcohol also abstracts the proton attached to the oxygen in the 2nd method discussed above. Well, this sure is a problem. If another alcohol abstracts the proton then it won’t be able to attack nucleophilically anymore. So, we need a base to speed up the reaction by introducing a base. Already there are many species in the solution, it would have been better if one of them also serves as the base. Well, let’s see if our blob can do it?
We have imposed too many conditions on the blob, let's summarise what those are:
· The blob should be a good electrophile (for phosphine to attack)
· The blob should be a good leaving group (for removal upon OH attack)
· The blob should also act as a base (To speed up the reaction)
Our strategy for OH removal:
The Blob: Alive or DEAD?
So far, we have successfully designed the reaction. And our entire quest boils down to one single question?
Does such a “blob” really exist?
It is much like in physics, where you know all the properties you function should have to describe the system, and then finding out if such a function exists or not.
The genius of Mitsunobu was to actually find that, Yes, such blob exists, and it’s DEAD.
Well, it’s not dead. That’s its name: Diethyl Azodicarboxylate (DEAD).
This molecule has all the properties a blob should have according to our deduction above, and therefore it would be able to remove the OH group as per our plan.
In the next post, I will discuss the mechanism of Mitsunobu reaction and the exact role of DEAD as the vital reagent.
In the next post, I will discuss the mechanism of Mitsunobu reaction and the exact role of DEAD as the vital reagent.