Walking in Mitsunobu's Shoes - Part 2: Your design at work
Mitsunobu reaction is 50 years old and still is of high importance to synthetic organic chemistry Although we have discussed it from the point of view of Remove-that-OH agenda, it was originally developed as just another ester formation reaction that results in inversion of stereochemistry. The success of the reaction was immediately followed in its use of natural product synthesis (Yaay !).
Let's then go into the reaction itself.
Substrate: primary/secondary alcohol (1)
Nucleophile: carboxylic acids, hydrazoic acid (2)
Reagents: Triphenylphosphine (3), Diethyl Azodicarboxylate (DEAD) (4)
Product: Ester/azide (13)
Stereochemistry: Inversion of configuration.
Let's then go into the reaction itself.
Reaction overview:
Substrate: primary/secondary alcohol (1)
Nucleophile: carboxylic acids, hydrazoic acid (2)
Reagents: Triphenylphosphine (3), Diethyl Azodicarboxylate (DEAD) (4)
Product: Ester/azide (13)
Stereochemistry: Inversion of configuration.
Mechanism:
I am going to break the reaction mechanism into steps, in a way that makes the connection with the previous post, where I presented how you can design the whole reaction from scratch. If you don't remember the sequential deduction it would be a good time to revisit.
So, assuming you have in mind the thoughts we had when we deduced the reaction, let's see if our design is really at work ...
Step 1:
The fastest reactions amongst organic species are acid-base reactions, due to their ionic nature. So, the first step would be the abstraction of the proton from the carboxylic acid (H-Nu) by the alcohol, and an equilibrium will be established between them. This is highly trivial and I won't bother to show it in the reaction scheme. But from the next step, we will need to focus, as the magic of 'DEAD' will be slowly revealed before our eyes.
First of all, clearly, phosphorus and nitrogen also has lone pairs and therefore can act as a base, but phosphorus is a pathetic base though a good nucleophile. And for DEAD, the lone pairs of the nitrogens are 'busy' in resonance with the carbonyl of the ester, which makes it unable to function as a base, at least in this form.
As triphenylphosphine bears a lone pair on it, it is a nucleophile, and if you look closely, you will see there is an electrophile waiting for it as well. Remember we needed our 'blob' to be an electrophile, that's exactly what happened because the nitrogens of the azo bonds of DEAD are in conjugation with the ester groups. This is like an α,β-unsaturated carbonyl electrophile.
So, PPh3 will attack one of the nitrogens and phosphorus would acquire the desired +ve charge on it. Yep, that's our first step.
Step 2:
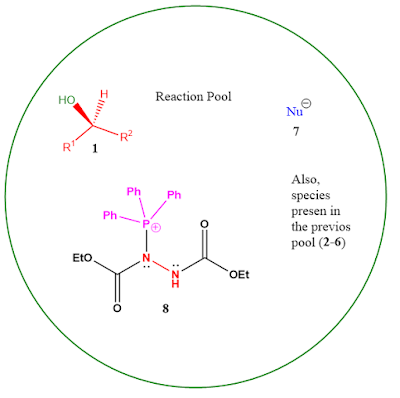
Now, there is a significant change in the reaction mixture. Notice that the nitrogens of the azo bond were double bonded before the attack of PPh3. But now one of them is sp3 hybridized and another has a partial double bond character due to resonance. So, the nitrogen in 6 bears a negative charge on it and therefore is considerably more basic than before. Thus it abstracts the proton of the carboxylic acid. If you remember, the carboxylic acid, H-Nu 2, and the alcohol were already in acid-base equilibrium, but due to the intervention of the base 6, the equilibrium shifts to the deprotonated alcohol side and hence we have both the nucleophile generated (Nu-) 7 and OH group available with its lone pair. This is where our designing strategy, the 'blob' needs to be a base pays off.
Step 3:
Again, we need to look for what species are present in the reaction pool/mixture currently (i.e., after 2nd step) to determine what's going to happen next. All the species previously present will still be there to some extent. But, forget everything, again something magical has happened at our DEAD complex 8. It is now an excellent leaving group. This is because upon leaving the minus charge on the sp3 nitrogen is going to be stabilized by resonance with the carbonyls of the adjacent ester group.
So, as per our design, the blob is ready to leave; but who's gonna attack? There is more than one nucleophile present in the solution and especially a clear-cut competition between the newly generated carboxylate 7 and the OH of the alcohol. But, the carboxylate is resonance stabilized and OH of alcohol is free; therefore, the alcohol 1 attacks the positively charged phosphorous center and the blob leaves.
Notice how the blob, species 10 plays the role of the base again. It abstracts the proton from the oxygen, which would have been done by another free alcohol molecule in the ordinary case. This is the benefit of having a symmetrical azo-dicarboxylate structure. Two times it can act as a base, once it generates the nucleophile and nextly it also catalyzes the reaction.
Note: This step is much like SN2 and the electrophilic site is a tertiary site, so it should occur relatively slowly.
Step 4:
Finally, lo and behold what we wanted. The oxygen of the alcohol is now attached to a phosphonium group with a +ve charge on the phosphorous 11. The whole thing from oxygen is an excellent leaving group. It's high leaving tendency is especially required if the nucleophile is a weak one like carboxylate (azide is relatively much stronger). So, the final step is now the removal of the oxygen along with its anchoring phosphonium group by the nucleophile present in the solution. This is a textbook- SN2 step and therefore an inversion in stereochemistry is expected.
Therefore, we have our product 13. In this case, it is an ester and in other cases, it may be something else, like an azide or something.
Comment: I have not specified the stereochemistry of intermediate chiral centers. Also, the lone pairs are not all shown. I hope these issues are understood.
Final Remarks:
I have discussed the whole mechanism step by step, elucidating what species are present in the reaction mixture at a time. In this way, you can understand the power of designing a reaction on your own. It may or may not work all the time, but it's worth a try. I must confess that this whole interpretation is my own (apart from the mechanism, which is generally accepted), and originally/historically probably is not the way in which it was discovered. But, nonetheless, it shows the power of a creative mind. A synthetic chemist is much like a chess player, who has to play and win by the rules of chemical transformation, but creative moves are always welcome, and sometimes serendipitous in the way of victory.








Comments
Post a Comment
Feel free to share your thoughts