Electrochemically Driven Desaturation: The Advent of Electrosynthesis
 |
| Electrochemically Driven Desaturation (EDD) |
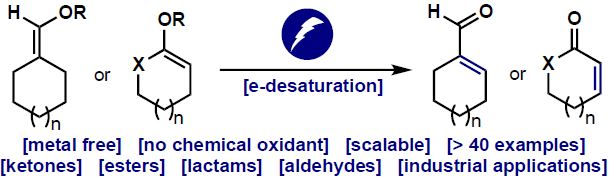
This very recent article by Baran group at Scripps Research Institute, California, is an excellent example of an electrochemically driven chemical transformation known as Desaturation (EDD). Desaturation is the reverse of hydrogenation of α,β-unsaturated carbonyl compounds where two hydrogen atoms are removed from the saturated molecule to generate an α,β-unsaturated moiety.
 |
| General idea of a desaturation reaction |
Many biologically active natural products contains such an α,β-unsaturated carbonyl moiety and this makes desaturation a very important technique because keeping a double bond unmasked throughout the whole synthesis is risky, as it is easily succumbs to electrophilic reagents. Thus, having the saturated 'version' throughout the whole synthesis and 'desaturating' it at the end is strategically more viable. (This makes the hydrogens a protecting group of the double bonds, with hydrogenation being the protection and desaturation being the deprotection step)
 |
| Natural Products containing α,β-unsaturated carbonyl scaffold |
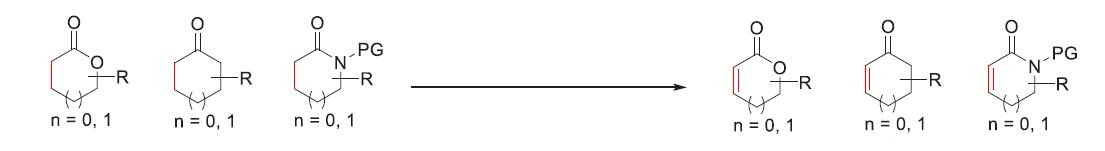
Early methods of desaturation used toxic selenium, sulfur-based reagents and stoichiometric oxidants, for this reaction and modern methods uses Pd-catalyzed or Cu-catalyzed transformations. Although, they give superior results compared to the previous methods, they are still limited by substrate scope and well, are metal-based.
The beauty of the current paper lies in two things, first this is the first desaturation reported that does not uses any transition metal based reagents (only thing close to it is NaSbF5 which is very cheap: $0.56/gram ) or any stoichiometric oxidants so to speak. And the second thing is even more exciting because it gives you a direct way to predict whether the desaturation will take place in your substrate or not.
 |
| Optimized reaction conditions for EDD |
The ability to predict the viability of desaturation occurring in your model substrate with a certain confidence makes this report very crucial because often in complex molecule synthesis, simple reactions do not work the way expected. So, it is best to know beforehand whether it will work or not while planning a retrosynthesis.
Baran group uses 1H-NMR shift as a guiding tool to predict the probability of desaturation occurring in a substrate. The ability to undergo anodic oxidation depends on the electron density at the double bond (which directly connects it to the oxidation potential) and thus, the 1H-NMR shift of the vinylic proton can act as a proxy for the electron density i.e., oxidation potential.
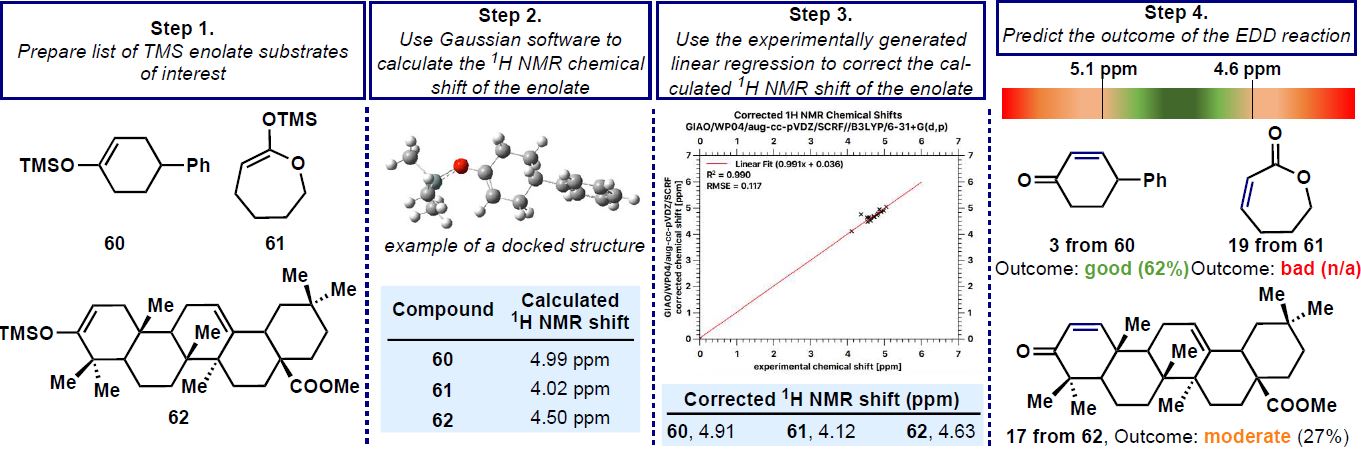 |
| Computational protocol for predicting the viability of EDD on model substrates |
The authors determined when the chemical shift of the vinylic proton lies in between 4.6 ppm to 5.1 ppm, the desaturation takes place with good yield. So, by using quantum chemical computations using Gaussian 16 software, the 1H-NMR shifts of the model substrate can be simulated and matched against the data to check the feasibility of the transformation.
(I should mention here that this is not the first time NMR shifts has been used to predict organic reactions. This so called Handy-method (pun again ?) describes a was to predict regioselectivity of cross coupling in polyhalogenated heteroaromatic compounds, which interestingly, Baran teaches in his Heterocyclic Course at Scripps)
The mechanism of the desaturation involves removal of two electrons by the anodic SET oxidation and removal of the β-proton by the base 1,3,5-collidine, in the order: electron, proton, electron as shown in the plausible mechanism proposed by the authors. The key intermediate is the radical cation generated by the first SET anodic oxidation of the silyl enolether substrate.
 |
| Proposed Mechanism of EDD |
Further attraction of this methodology lies in the fact that strict exclusion of air and moisture is not necessary for this protocol, however, doing so improves the yield even more. The extraordinary paper is accompanied by a detailed supplementary information with pictures of the reaction setups and TLC plates (characteristic of a Baran paper). And I was amazed to see a troubleshooting FAQ section in it that deals with some common problems and questions that one might face while doing the reaction. This is what makes a reaction protocol robust and reproducible for the common mass (well, in scientific community at least) by making electrochemistry less alien and recognizing it as a greener approach towards synthesis. I hope that day is now closer when electrosynthesis will be a part of regular organic synthesis projects in labs, including fluent use in complex natural product synthesis.
Comments
Post a Comment
Feel free to share your thoughts