Shortest Total Synthesis of Prostaglandin
Prostaglandins are a class of molecules that helps regulate many physiological activities inside the body. Derivatives of prostaglandin and analogues are used heavily in pharmaceuticals for various uses. an
analogue of PGF2α, latanoprost, is used in the treatment of glaucoma, and is a billion-dollar drug as of 2010. However, their synthesis requires many steps and generates a lot of waste.
Back in 1969, E J Corey developed a 17 steps synthesis of Prostaglandin F2α and that was the preferred route for a long time, simply because there was no other better route. But in 2012, Varinder Aggarwal and his group reported a 7 step synthesis of PGF2α which uses a proline catalysed aldol reaction cascade.
 |
| Aggarwal's retrosynthesis of PGF2α |
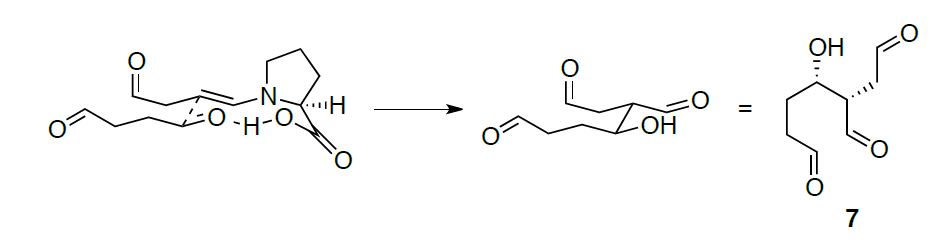
By using succinaldehyde as a simple starting material, the crucial intermediate 7 can be accessed, which contains 2 of the 4 stereocenters of PGF2α. This incredible transformation is a proline-catalyzed enantioselective aldol condensation cascade that actually consists of three steps happening one after another in the same reaction sequence (the very definition of a cascade). The first step is the enantioselective intermolecular aldol reaction via proline enamine to give the intermediate 10 which then forms a acetal 11 that undergoes a intramolecular aldol condensation to generate 7.
 |
| Steps in the proline-catalyzed Aldol Cascade |
The stereoselection can be explained (or planned, in this case) by the Houk-List model of proline organocatalysis, and the same is shown in a simplified form below.
However, only intelligent designs doesn't always lead directly to success, as in this case. Aggarwal group faced a difficult problem in optimizing the aldol cascade. The product 7 is only one of the products that can form via the shown pathway and numerous other competitive pathway are possible where oligomeric aldol products are obtained. Especially when only proline is used as catalyst, the oligomeric products prevails. To diminish the formation of these unwanted side-products, the authors employed another catalyst, [Bn2NH2][OCOCF3]. This catalyst was shown to prevent the original proline-catalyzed aldol reaction. So, by adding the 2nd catalyst after some time since the first catalyst is added, further aldol reactions can be prevented and the reaction can be arrested till the desired point.
 |
| Optimized reaction conditions of the cascade |
Thus, the authors were able to synthesize the desired cascade product 7 as the major product. The enantioselectivity was great (e.r. = 99:1) although the yield was low (14%). However, the benefit of completing the whole synthesis in only 7 steps (5 steps after the aldol cascade) outweighs the disadvantage of having such a low yield step.
At this point, I should mention that although the yield of the aldol cascade in this paper is 14%, in another paper in 2018, the group has modified the reaction conditions to increase the yield to 29%.
 |
| 7 step enantioselective synthesis of PGF2α |
Despite the low yield cascade, this is an improvement over the previous total synthesis by literally an order of magnitude (7 vs 17 steps). The overall yield is 3.3%, and 1.9 grams of PGF2α was prepared following this route.
This landmark synthesis shows the rising potential of organocatalysis in synthesis of natural products, They provide metal free, greener approaches with very high stereoselectivity. However, it is yet to recover from issues of low yield and high-catalyst loads. I am hopeful the day will come when even total synthesis will be dominated by organocatalyzed cascades that will enable high yielding, short routes to the desired target molecules. And that day is probably not too far away.

Comments
Post a Comment
Feel free to share your thoughts