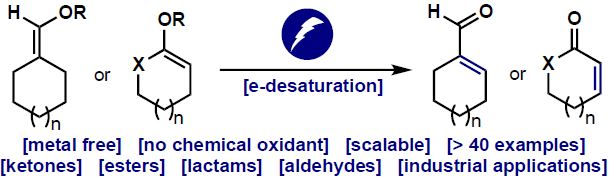
Mechanistic Steps in Organometallic Chemistry
Robert Grossman mentions in his book "The Art of Writing Reasonable Organic Reaction Mechanisms" that if you cannot classify an alleged pericyclic step in your mechanism, then probably that step is a fictional one. Like many other things on the book, I have found this advise very helpful and often saved me from committing a grave mistakes on paper. I figured this 'rule' must also extend to other cases of reaction mechanisms and not only to pericyclic reactions. I have noticed it is easy to identify things if we name them. So I am gonna call this the rule of classified mechanisms . Don't judge, no one said I am an expert on naming things. (I originally thought of calling it the "Grossman's Rule" but that is already exists in the book) As organometallic chemistry is synthetically versatile and widely used in research, I planned to have a small page consisting all the valid reaction mechanism steps at one place. So any step you write while doing a o...